Question
A student is given a buffer that contains [HA] and [A-]. The student is given the...
A student is given a buffer that contains [HA] and [A-]. The student is given the concentration of [HA] and need to solve for the concentration of [A-]. Should they titrate with HCl of NaOH? Explain your reasoning.
Homework Answers
Answer #1

Not the answer you're looking for?
Similar Questions
1. A student is given a buffer that contains [HA] and [A- ]. The student is...
1. A student is given a buffer that contains [HA] and [A- ]. The
student is given the concentration of [HA] and need to solve for
the concentration of [A- ]. Should they titrate with HCl or NaOH?
Explain your reasoning
2. At an equivalence point of 35.0 mL, how many moles of [HA]
are present in the buffer if you titrated with 0.125 M NaOH?
3. A student titrated 25.0 mL of a buffer with 0.126 M HCl. The...
The problem statement, all variables and given/known data Group#1: Buffer pH = 4.00 Group#2: Buffer pH...
The problem statement, all variables and given/known data
Group#1: Buffer pH = 4.00
Group#2: Buffer pH = 4.35
Group#3: Buffer pH = 4.70
Group#4: Buffer pH = 5.00
Group#5: Buffer pH = 5.30
Group#6: Buffer pH = 5.60
1. Explain which group should havethe BEST OPTIMAL BUFFER (see
choices above).
2. Explain which group has a buffer that has the HIGHEST
BUFFERING CAPACITY AGAINST NaOH.
3. Explain which group has a buffer that has the HIGHEST
BUFFERING CAPACITY AGAINST HCl....
1.) A student titrated 25.0mL of a buffer with 0.126 M HCL. What is the concentration...
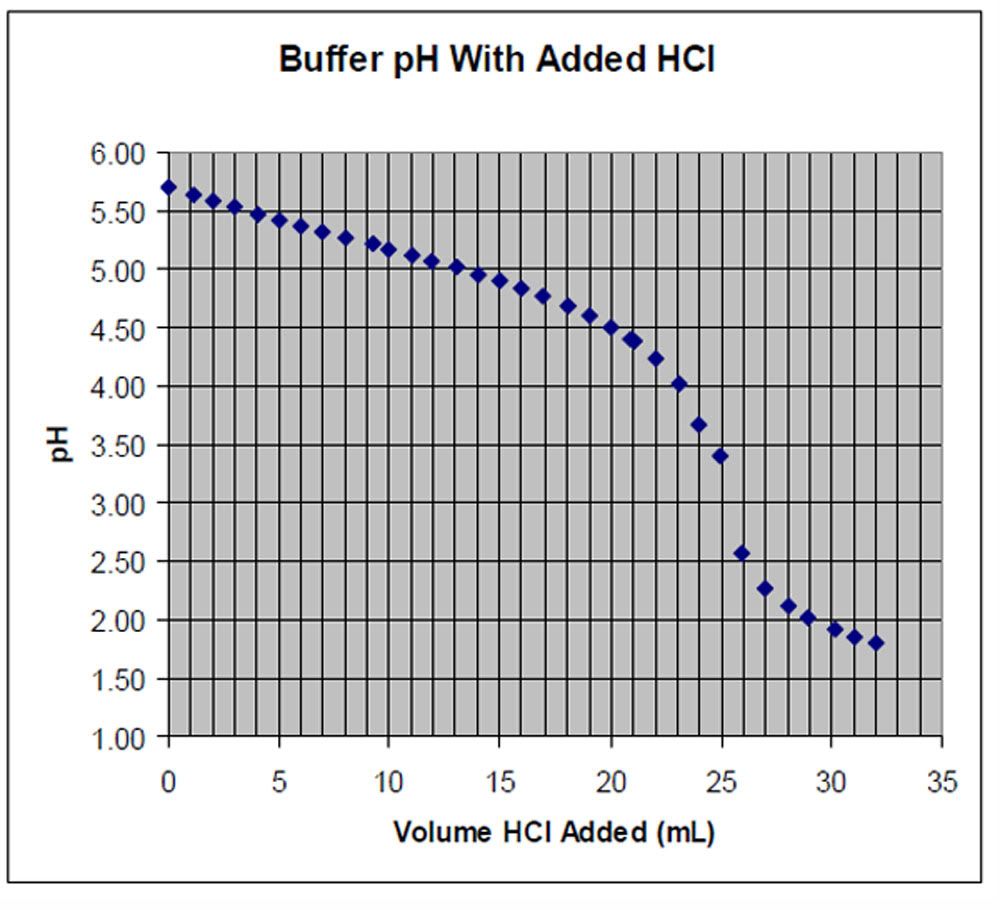 1.) A student titrated 25.0mL of a buffer with 0.126 M HCL. What
is the concentration of the weak base in the buffer? Document your
logic(do not use M1V1=M2V2)
http://i1190.photobucket.com/albums/z451/chemistrypictures/chart1.jpg
2.) The student then titrated 25.0 mL of the same buffer with
0.115M NaOH. The observed titration curve is shown below. What is
the concentration of the weak acid in the buffer?
http://s1190.photobucket.com/user/chemistrypictures/media/graph1.jpg.html
3.) What is the buffer strength?
4.) If the pH of the buffe (before it is titrated)...
1.) A student titrated 25.0mL of a buffer with 0.126 M HCL. What
is the concentration of the weak base in the buffer? Document your
logic(do not use M1V1=M2V2)
http://i1190.photobucket.com/albums/z451/chemistrypictures/chart1.jpg
2.) The student then titrated 25.0 mL of the same buffer with
0.115M NaOH. The observed titration curve is shown below. What is
the concentration of the weak acid in the buffer?
http://s1190.photobucket.com/user/chemistrypictures/media/graph1.jpg.html
3.) What is the buffer strength?
4.) If the pH of the buffe (before it is titrated)...You are given a buffer that contains a weak base whose concentration is 0.600 M and...
You are given a buffer that contains a weak base whose
concentration is 0.600 M and its conjugate weak acid whose
concentration is 0.260 M. The volume of this solution is 0.375 L.
If 0.080 L of an HCl solution with a concentration of 1.10 M is
added to this solution, what will the pH of the combined solution
be? The pKa of the weak acid is 4.67.
1. A buffer that contains 0.37 M of an acid, HA and 0.48 M of its...
1. A buffer that contains 0.37 M of an acid, HA and 0.48 M of
its conjugate base A-, has a pH of 3.76. What is the pH
after 0.02 mol of NaOH are added to 0.71 L of the solution?
2. Calculate the pH during the titration of 30 mL of 0.25 M
HNO3(aq) with 0.18 M NaOH after 18 mL of the base have
been added.
Your assigned pH is: 4.6 You are to assume that the buffer is made up by...
Your assigned pH is: 4.6
You are to assume that the buffer is made up by mixing
volumes of 0.100 M acetic acid and 0.100 M sodium acetate
solutions.
Calculate the volume of 0.100 M acetic acid required
to prepare 60.0 mL of a buffer of pH 4.6.
35.0 mL
1 ptsYou are correct.
Your receipt no. is 168-3130 Previous Tries
Calculate the volume of 0.100 M sodium acetate
required to prepare 60.0 mL of a buffer of pH 4.6.
25.0...
Given that Buffer A contains 200.0 mL 0.050 M HOCl and 400.0 mL 0.030 M NaOCl,...
Given that Buffer A contains 200.0 mL 0.050 M HOCl and 400.0 mL
0.030 M NaOCl, calculate
1. The pH of the buffer solution.
2. The pH of the solution after adding 10.0 mL 0.50 M HCl.
3. The pH of the solution after adding 20.0 mL 0.40 M NaOH
A student needs to prepare a lactic acid buffer at a pH of 4.85. The student...
A student needs to prepare a lactic acid buffer at a pH of 4.85.
The student has available lactic acid (pKa = 4.20), a 0.830 M KOH
solution and a 0.830 M HCl solution. Will the student need to add
KOH or HCl to the lactic acid in order to prepare the buffer?
Also
Calculate the volume (in mL) of the solution that should be
added to 3.166 g of lactic acid (FW 122.12 g/mol, pKa = 4.20) to
give...
A buffer with a pH of 3.90 contains 0.15 M of sodium benzoate and 0.30 M...
A buffer with a pH of 3.90 contains 0.15 M of sodium benzoate
and 0.30 M of benzoic acid. What is the concentration of [H ] in
the solution after the addition of 0.056 mol of HCl to a final
volume of 1.4 L? Assume that any contribution of HCl to the volume
is negligible. WHAT IS CONCENTRATION OF HCL ADDED TO BUFFER??
question 1.Your TA assigns you two monoprotic( one proton per molecule) acids HA and HB. You...
question 1.Your TA assigns you two monoprotic( one proton per
molecule) acids HA and HB. You are given 43.5 mL of HA solution in
the first flask. A second flask contains 37.2 mL of HA and enough
HB solution is added to reach a final volume of 50.0mL. You titrate
the HA solution, in the first flask, with 87.3mL of 0.0906 M NaOH.
What is the molarity of the acid HA? ( Write answer to two decimal
places, ex. 1.25...
ADVERTISEMENT
Need Online Homework Help?
Get Answers For Free
Most questions answered within 1 hours.
ADVERTISEMENT
Active Questions
asked 6 minutes ago
asked 20 minutes ago
asked 33 minutes ago
asked 43 minutes ago
asked 52 minutes ago
asked 1 hour ago
asked 1 hour ago
asked 1 hour ago
asked 1 hour ago
asked 1 hour ago
asked 1 hour ago
asked 1 hour ago